Thin, soft electronic systems that stick onto skin are beginning to transform health care. Millions of early versions1 of sensors, computers and transmitters woven into flexible films, patches, bandages or tattoos are being deployed in dozens of trials in neurology applications alone2; and their numbers growing rapidly. Within a decade, many people will wear such sensors all the time. The data they collect will be fed into machine-learning algorithms to monitor vital signs, spot abnormalities and track treatments.
Medical problems will be revealed earlier. Doctors will monitor their patients’ recovery remotely while the patient is at home, and intervene if their condition deteriorates. Epidemic spikes will be flagged quickly, allowing authorities to mobilize resources, identify vulnerable populations and monitor the safety and efficacy of drugs issued. All of this will make health care more predictive, safe and efficient.
Where are we now? The first generation of biointegrated sensors can track biophysical signals, such as cardiac rhythms, breathing, temperature and motion3. More advanced systems are emerging that can track certain biomarkers (such as glucose) as well as actions such as swallowing and speech.
Small companies are commercializing soft biosensor systems that measure clinical data continuously. These include Vital Connect in San Jose, California; iRhythm in San Francisco, California; MC10 in Lexington, Massachusetts; and Sibel Health in Evanston, Illinois. For example, iRhythm’s single-use Zio patch monitors electrical pulses from the heart for 14 days, and is more effective than intermittent hospital check-ups at detecting abnormal rhythms4. But it is bulky and temporary, and the data must be downloaded after use, rather than transmitted in real time.
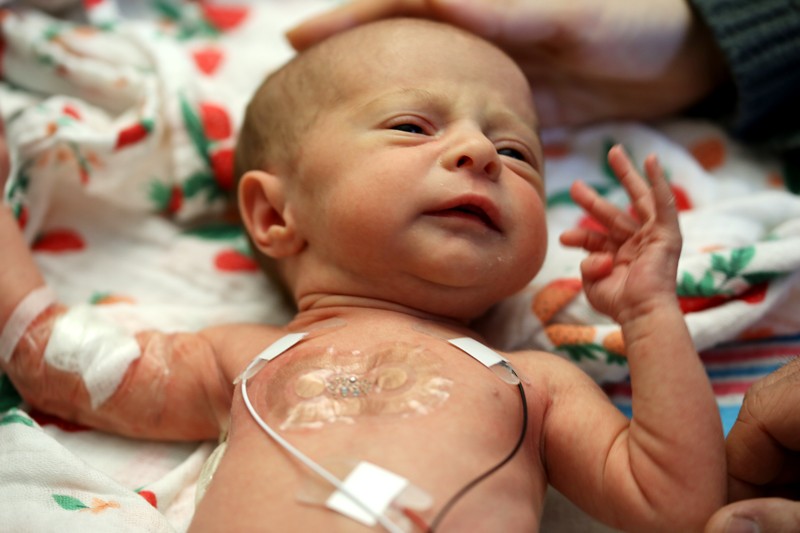
More advanced sensors from our labs are undergoing clinical trials in Chicago, Illinois5. These include even smaller sensor networks for heart rate, respiration and temperature. They can transmit data wirelessly, and are soft enough to place on the chests of premature babies without damaging their fragile skin6. There is no need for nurses, doctors or parents to disconnect a forest of wires when they want to pick up a baby. Similar systems might sense pressure and temperature in people who have had limbs amputated, at the interface between a limb socket and prosthesis.
Many challenges must be overcome to make wearable sensors fit for widespread use. Innovations in materials, devices and circuit designs must make soft biosensors even smaller, thinner, lighter and less power-hungry. The accuracy, precision and range of measurements must improve. And regulation, costs, usability and data security require attention.
Here, we outline the priorities for action.
To-do list
Biomarkers. All the flexible sensor systems approved by the US Food and Drug Administration (FDA) so far collect biophysical signals. Biochemical signatures, such as glucose or hormone levels, are hard to glean without puncturing the skin with needles.
Some emerging devices collect fluid by inserting a filament into the skin. And detecting chemicals in sweat is a promising alternative7. Sweat contains many indicators relating to cell health and organ function (such as electrolytes), the immune system (cytokines) and drug interactions (metabolites). Sweat sensors are being developed that capture chloride, glucose, lactate, urea, creatinine, alcohol, pH and even heavy metals. Quantifying protein and hormone levels in sweat would increase these sensors’ applicability further.
Still, sensors need to be able to collect and analyse sweat without it becoming contaminated or degrading, and they will also require new chemical tests and types of assay.
Tools. Imaging and spectroscopy capabilities would allow for real-time assessments of the body. Examples are optical coherence tomography, confocal microscopy, Raman spectroscopy and two-photon excitation microscopy. If such systems could be miniaturized, they could diagnose skin tumours without the need for a biopsy sample or surgery. They are currently still expensive, bulky and wired.
Therapies. Interfaces that create skin sensations, such as vibrations, might enhance rehabilitation, notably with speech and motion therapies. Drugs could be delivered through skin patches, as they are already for motion sickness (scopolamine), pain (fentanyl), contraception (norelgestromin and ethinylestradiol) and high blood pressure (clonidine). The release could be triggered electrically, acoustically or thermally, for example, by applying heat to a polymer pocket. Sensors could also deliver electrical or thermal stimulation to treat neurological disorders or modulate pain.
The single-use Zio patch from iRhythm in San Francisco, California, can monitor heart rate continuously for two weeks to detect irregularities.Credit: iRhythm Technologies
Implants. Soft sensor systems could be used inside the body. A thin, flexible implant might be wrapped around the heart or spine to monitor and stimulate it. Demonstration versions of thin, flexible technologies that track the electrical activity of the brain have been tested in mice, cows and non-human primates. Practical challenges include developing biocompatible materials and manufacturing ultra-thin layers that protect the electronics for years or decades. Some patches might melt away harmlessly after they have done their job, just as a wound heals.
Materials and design. There is work to be done to make devices less perceptible to wearers. Today’s patches typically include ultra-thin silicon electronics in a matrix of silicone elastomers. In future, organic polymers could be used to make biosensors that repair themselves. And the soft materials will have functions of their own, perhaps being antimicrobial or able to change colour if a biochemical is detected. Power could be harvested from body motions or changes in heat or blood flow rather than from batteries8.
Data. Combinations of sensors need to be designed to suit certain conditions. For example, for Parkinson’s disease, a single sensor on the hand is enough to detect tremors9. But in people who have had a stroke, characterizing how hard their foot hits the ground when walking, how strongly they swallow or how soundly they sleep would require additional sensors and data outputs — from accelerometers, gyroscopes, microfluidic sensors, and electrocardiographs and electromyographs (which measure electrical activity in the heart and muscles, respectively). To improve data quality, these sensors should be sited on the best places on the body to collect information — for example, electrocardiogram signals should be recorded on the chest, not the wrist. Gait is better assessed with sensors on the ankles. Noise will need to be filtered out, and decisions will need to be made about whether it is better to stream all of the data to the cloud or process some of them on the chip and transmit only key parameters or insights extracted from the base data, in the form of warnings or notices.
Interpretation. Digital dashboards need to be developed to allow physicians and patients to track outputs, log changes and make clinical decisions. Machine-learning models need improvement, for example to predict how long it will be until a patient is discharged from hospital or is able to walk or feed themselves safely without assistance. Long-term monitoring in the community would help physicians to assess the evolution of stroke recovery, Parkinson’s disease and other disorders.
Behaviour. More needs to be learnt about how patients use biosensors in their everyday lives. If people are to wear the devices for weeks or months, the patches will need to look acceptable, and ideally attractive. They should be comfortable and maintain good contact with the skin during washing or exercising. Although some sensors are now small enough fit on a fingernail and thin enough not to show through clothing, they will need to become yet smaller and thinner.
Clinical practice
Bringing these technologies to patients will take action on three more fronts: validation, regulation and data protection.
To speed up their entry into the clinic, soft biosensors must target unmet medical needs, such as mental-health monitoring in the home10. Changes in vital signs and in neuroendocrine, neurotropic and inflammatory biomarkers could yield insights that are unavailable to clinicians today. Signs of social isolation and loneliness might prompt a visit from a carer or a call from a loved one.
Wireless health monitoring could also revolutionize health care in countries where infrastructure is lacking. We will trial our biosensors in maternity clinics in several African countries, including Zambia, Kenya and South Africa, later this year, in partnership with the non-profit organizations the Bill & Melinda Gates Foundation and Save the Children. The patches will track physiological data such as physical activity, blood pressure and respiratory rate in women and their babies during pregnancy, warning of complications such as fetal hypoxia or an impending haemorrhage.
Regulatory approval is crucial, and challenging to obtain. Hardware is largely covered by existing frameworks; algorithms are not. But there are encouraging signs that software applications can be regulated. In the past few years, the FDA has approved machine-learning technology for the diagnosis of diabetic retinopathy, the first pill with an embedded sensor (Abilify MyCite) and an app to treat opioid-use disorder (reSET-O). The FDA’s pre-certification programme allows medical software from certain trusted developers to be deployed before formal evaluation.
Regulations must adapt quickly, as the boundaries between devices, data, software and therapeutics continue to blur. Special attention should be paid to clinical areas of highest need and minimal risk — there are some such within rare diseases, paediatrics, women’s health and gerontology.
Data security must be a top priority, particularly for patient information. The US Health Insurance Portability and Accountability Act established guidelines for the confidential handling of patient information in 1996. But this was well before the explosion in mobile devices and wearable sensors. New frameworks are needed. Patients must own their own data. And great care must be taken to ensure that companies do not exploit medical data for commercial gain without approval, or drive a division between those who can and cannot access this technology.
Given the poor track record of private companies in protecting consumer privacy, leadership at both the national and international level is needed. Policies must prevent employers and insurers from discriminating against people with particular data profiles, much as the US Genetic Information Nondiscrimination Act of 2008 protects workers. Deviations should be met with serious financial and legal punishments11.
It remains to be seen how these sensor systems will be paid for, and how doctors will be reimbursed for interpreting and acting on the data. Still, health-care funders should champion biointegrated sensor systems because they can potentially improve the quality of care and lower costs. This fits with the move towards value-based care in the United States, where health-insurance companies and government plans such as Medicare are selecting treatments on the basis of efficacy rather than simply reimbursing services.
Road ahead
Technical progress will require close collaborations between materials and device engineers, data scientists and medical professionals. Users and carers need to be more closely involved.
Interdisciplinary funding from government sources, corporate investments and charitable foundations will be essential for collecting proof-of-concept data before devices can be commercialized. For example, the Michael J. Fox Foundation in New York City has grant programmes focused on wearable technologies in global health.
Companies need to improve manufacturing processes for devices that combine hard skeletal components and soft tissue-like materials. Yields and throughputs need to be improved to assure quality and lower costs. Automated tools are needed to design the layout and topology of circuits and mechanical components.
The effort will be worth it: bio-integrated sensors have the potential to transform nearly every aspect of medicine.
[“source=nature”]